Safe use of recycled plastics for cosmetics
HDPE bottles for Personal Care and Cosmetic products (Cosmetics) are an attractive segment for the use of post-consumer recycled (PCR) materials. The availability is poor for materials that have good enough mechanical properties and purity. The latter can cause odour and migration of substances which needs safety documentation. Can a 3-layer structure be the solution?
By Jorunn Nilsen, Norner
HDPE bottles for Personal Care and Cosmetic products (Cosmetics) are an attractive segment for the use of post-consumer recycled (PCR) materials. The availability is poor for materials that have good enough mechanical properties and purity. The latter can cause odour and migration of substances which needs safety documentation.
Can a 3-layer structure be the solution?
Cosmetic regulation
In contrast to food contact applica- tions, cosmetics do not have a defined process for signing off safety and regulatory compliance for the use of PCR materials.
Instead, the EU Cosmetics Regulation (EC) No. 1223/2009 says that the cosmetic product made available on the market must be safe for human health when used under normal or reasonably foreseeable conditions of use. A safety assessment that involves information about the packaging material must be performed and included in the required Product Information File (P.I.F.).
Safety assessment
The introduction of PCR materials in the packaging increases the complex- ity of this safety assessment. Due to this, the qualification of PCR materials for use in Cosmetic applications is a high priority for packaging producers and brand owners.
The amount of PCR material that can be used in cosmetic packaging such as HDPE bottles depends on the material quality and purity. In single-layer bottles, it is common to use blends of PCR and virgin HDPE ma- terial to obtain a safe result. Another common method is to use PCR in the core layer in multi-layer bottles. Then there is a virgin food contact-compli- ant material in the inner layer of the bottle, in contact with the product.
Strategic investments
To support the global transition from virgin fossil-based materials to recycled polymers for various applications, Norner has enhanced its competence and resources. We have invested strategically in people and equipment to provide required solutions for the circular economy goals set by the authorities, brand owners, and the industry, including:
- New analytical techniques for PCR purity assessment (GC/ MS-ODP, GC/FID, LC/QTOF).
- Recycling Pilot for PCR upgrade by better extrusion
- 7-layer cast and blown film large pilot line
- 3-layer Extrusion Blow Moulding (EBM) commercial scale bottle production unit.

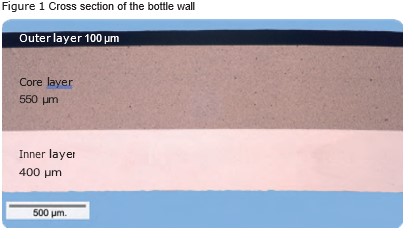
Study of 3-layer bottles
Norner has made a large case study where migration testing and screening of non-intentionally added substances (NIAS) have been performed on 3-layer bottles intended for cosmetic packaging. The following paragraph presents the main results. The test bottles had PCR material in the core layer, a 400 µm inner- and a 100 µm outer layer. See Figure 1, which shows the layer distribution of the bottles.Cosmetic products a have long shelf life (typically > 2 years). Due to this, it was decided to use 10 days at 60 °C as migration conditions. Two simulants were selected: 95 % and 50 % ethanol. NIAS screening is often performed with 95 % ethanol as a simulant, and it is well known that 10 days at 60 °C are tough conditions.
50 % ethanol is a milder simulant, and a lower level of migration could be expected. A commercial PCR grade and a virgin HDPE bottle grade were selected for the study. Reference bottles with 100 % virgin material was also tested. Migration from the raw material (pellets) were compared to the results from the bottle migration under the same conditions.
Migration test results
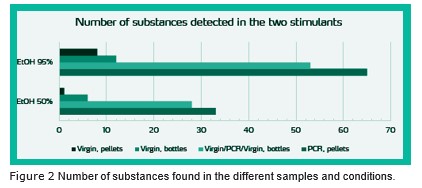
The migration tests were based on EN1186, and for the NIAS screening, Gas Chromatography with Mass Spectrometric detector (GC-MS) was used. See Figure 2, which summarises the results. A total of 69 different substances were detected in the analyses at concentra- tions ³ 5 ppb, from the PCR pellets.
- This includes polyolefin oligomeric saturated hydrocarbons (POSH), which are shorter polymer chains always present in HDPE.
- Some intentionally added substances (IAS), i.e. additives, were identified.
- The main part of the substances was contaminants/NIAS.
From the virgin materials, very few substances were found. There were more substances from bottles than from pellets, in both simulants. For all materials, we found fewer substances with 50 % ethanol compared to 95 % ethanol. We found more substances in the bottles with PCR core layer than in the virgin bottles.
- With 95 % ethanol, more than 40 substances have migrated through the 400 µm virgin inner layer
- In 50 % ethanol, only half of these substances had migrated through the virgin layer.
- The amount of migration per sub- stance was significantly less when the bottle had an inner virgin
The number of substances was high- est for migration from the PCR pellets. Some of these did not migrate through the virgin inner layer of the bottles.
Conclusion
Based on the results, we can conclude that a virgin plastic layer acts as an inhibitor to migration, and even if it is not an absolute inhibitor, it reduces the risk and increases consumer safety. However, in order to remain safe, the migration and safety assessments need to be made.
